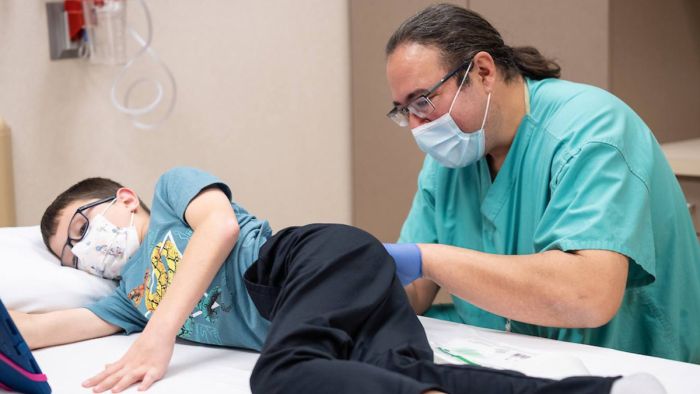
Xavier and Rose Menke are part of a new generation of children diagnosed with spinal muscular atrophy (SMA) who have a brighter future thanks to new treatment breakthroughs.
The neurology team at Gillette Children’s is one of the leading pediatric facilities involved in SMA drug trials and neuromuscular disease research and treatment.
Research, done in part at Gillette, is now providing more positive outcomes for children diagnosed with this rare, hereditary, and often-fatal disease affecting muscle strength and movement. In the past, children diagnosed with the most common type of SMA (type 1) had a lifespan of less than two years without treatment.
Gillette pediatric neuromuscular neurologist, Randal Richardson, MD, MMS, is a leading SMA researcher and has been on the forefront of developing these new treatments.
Parents Appreciate the Gillette SMA Care Team
For the past six years, parents Carrie and Tony Menke have been making the four-hour drive from Sioux Falls, South Dakota, to see Dr. Richardson and his neurology team.
“We just love Dr. Richardson. He’s been wonderful for Xavier, who is 10, and he’s cared for our now 3-year-old daughter, Rose, even before she was born. We totally feel it’s worth the extra effort to come to Gillette in St. Paul for their treatment.”
Carrie Menke
Hope For a New Generation of SMA Patients
Dr. Richardson and his team were key contributors in the 2016 EMBRACE clinical trial that led the Food and Drug Administration (FDA) to approve Spinraza® (nusinersen) for SMA treatment.
Spinraza is hailed as the first real treatment for SMA. It works by targeting the SMN2 gene, causing it to make a more complete protein. According to Cure SMA, once a patient receives four loading doses within the first two months of treatment, they receive a maintenance dose every four months for life.
Gillette and Spinraza Help Xavier
The approval of Spinraza came at just the right time for Xavier Menke, who was born in November 2013.
“Xavier was officially diagnosed with SMA in 2017,” Carrie says. “It was just a few months after the first SMA treatment became available.”
His parents report Xavier was born healthy, but they started noticing symptoms when he was around 2 years old.
“Something was off,” Carrie says. “He had been toddling around and talking from an early age. He walked at 1 and was right on track. Then when he was about 2, he would often fall and using the stairs became a challenge. He started to decline rapidly.”
As Xavier’s symptoms became more severe, the Menkes sought advice from their family pediatrician who recommended that Xavier go to Gillette for an evaluation.
Gillette Experts Make a Difference in Treatments
Xavier initially received the four loading doses of Spinraza and he comes to Gillette several times a year, during which Dr. Richardson monitors him and gives him lumbar punctures of the medication.
“Nusinersen (Spinraza) represents the first real opportunity to shift the focus in neuromuscular care from diagnostics and symptom-focused treatments to true medical treatment for a previously untreatable genetic disease,” Dr. Richardson says.
Randal Richardson, MD, MMS
“Embracing this early and aggressively meant that I peddled a bit in hope, a word we should use more commonly in medicine,” Dr. Richardson says. “Once the new natural history of nusinersen-treated SMA became established…it turned out that our hopes were indeed congruent with reality. Xavier proved to me that it is OK to hope and strive for a better tomorrow.”
Gillette and Zolgensma Help Rose
Xavier’s sister, Rose, is part of a new generation of children diagnosed with SMA. Before she was born, Rose was tested in utero to detect if she had SMA. The amniocentesis test came back positive for SMA, and a neonatologist was present at Rose’s birth on Dec. 8, 2019.
“A week after Rose was born, we traveled to Gillette to see Dr. Richardson,” Carrie says.
When she was just one month old, Rose received the second SMA treatment approved by the FDA. Dr. Richardson and his team administered Zolgensma, which is the first gene therapy approved to treat neuromuscular disease.
The FDA approved Zolgensma in May of 2019. According to the Cure SMA website, Zolgensma is given through an intravenous (IV) infusion that takes about one hour. It is a one-time treatment to treat children with SMA who are younger than 2 years of age.
So far, Rose shows no sign of the symptoms associated with SMA. She walks, runs, jumps, and is a ball of positive energy. Unlike her older brother, Xavier, Rose does not need continued lumbar punctures or as many visits to Gillette.
Dr. Richardson says Xavier and Rose are examples of how important it is for children to be diagnosed as early as possible. The Menke children also highlight the progress in SMA care.
“Rose’s continued lack of symptoms is most certainly due to Zolgensma,” Dr. Richardson says.
Gillette Helps to Ensure SMA Newborn Screenings
Thanks to the work of Dr. Richardson and his team, in March 2018 Gillette was instrumental in ensuring children born in Minnesota are screened for SMA at birth. Because of this, Minnesota was among the first states in the country to screen for SMA. This is important because the sooner a child is diagnosed and treated, the less damage there is from SMA.
Gillette is in the Cure SMA Care Center Network and is active in maintaining and enhancing the Cure SMA Clinical Data Registry, which is hoped to evolve to maximize both medical and supportive therapies for SMA.
Children like Xavier and Rose Menke are examples of how Gillette’s focus on research and its unwavering commitment to cutting edge care can have an impact on a child’s life.
Join Our Partners in Care Community!
Subscribe to Partners in Care Journal, a newsletter for medical professionals.
Subscribe Today Home Page
Home Page