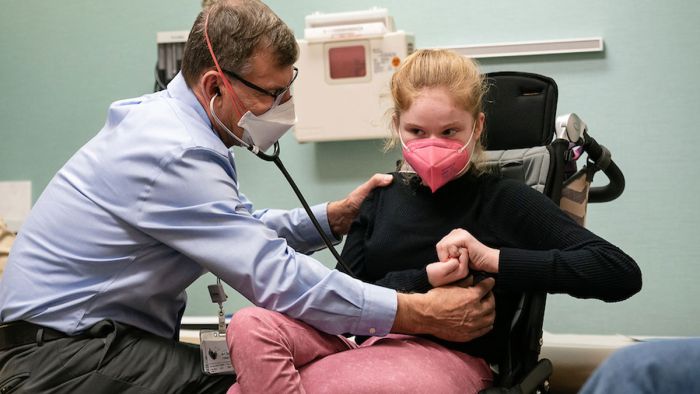
There’s renewed hope for patients diagnosed with Rett syndrome a year after the FDA approved Daybue (trofinetide) as the first approved treatment to help people diagnosed with the rare neurodevelopmental disorder.
Most infants with Rett syndrome are girls who appear to develop typically until age 2 or 3. Children will stop gaining developmental skills, such as walking or talking and might lose skills they had achieved.
About 50 Gillette Children’s patients diagnosed with Rett syndrome have been taking Daybue (trofinetide) for about a year.
“These patients are improving incrementally, and we see improvements in communication skills, breathing and overall quality of life,” says Arthur Beisang, MD, co-director of the Gillette Children’s Rett Syndrome Center of Excellence.

Dr. Beisang and Dr. Feyma are the co-directors of the Gillette Children’s Rett Syndrome Center of Excellence.
Gillette helps patients and parents have a voice
Gillette is one of 18 institutions across the U.S. that is designated as a Center of Excellence by the International Rett Syndrome Foundation (IRSF). Dr. Beisang and pediatric neurologist Tim Feyma, MD, co-direct the Gillette Children’s Rett Syndrome Center of Excellence.
“Gillette really became a center of excellence because parents of kids who have Rett came to us and said, ‘we need a voice in the medical field.’ It is really the parents and the Gillette leaders who helped us become a strong place for rare disease care,” Dr. Feyma says.
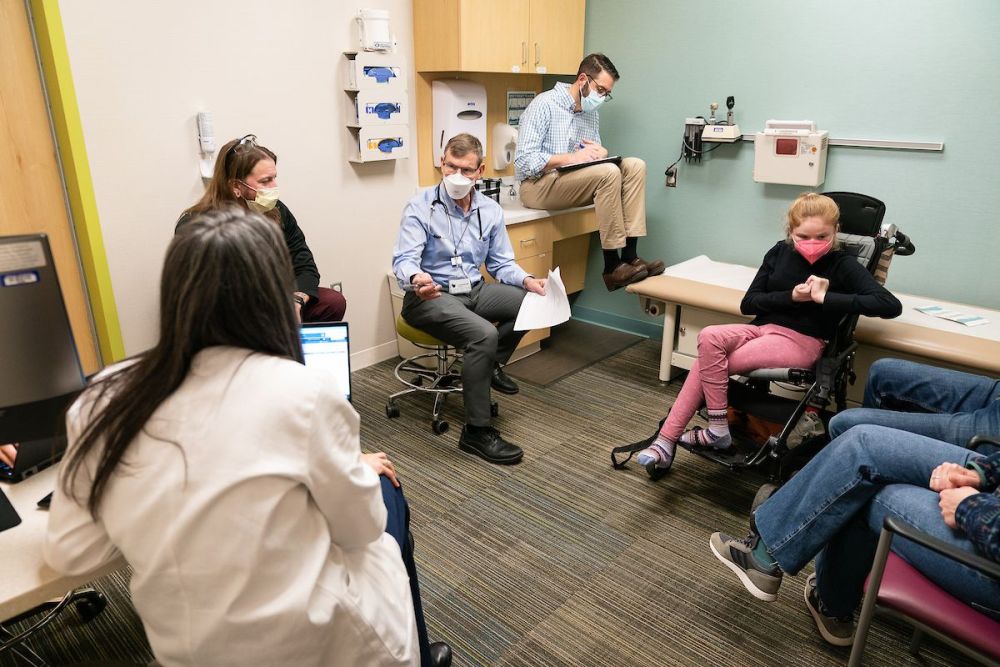
Gillette's rare disease care includes a variety of providers all in one clinic appointment to collaborate and ensure the best care for patients.
Gillette makes "a world of difference" for rare disease care
“What makes rare disease care at Gillette stand out,” Dr. Beisang says, “is we have a core group of providers who can handle the neurological needs, the pediatric needs, and the rehabilitation needs of a patient.”
Dr. Feyma agrees and says, “A lot of places have multidisciplinary clinics. Other places can offer care but not the kind of collaboration we have. At Gillette the providers can come to you during your clinic visit. Having my colleagues together in a clinic room can make a world of difference for these kids.”
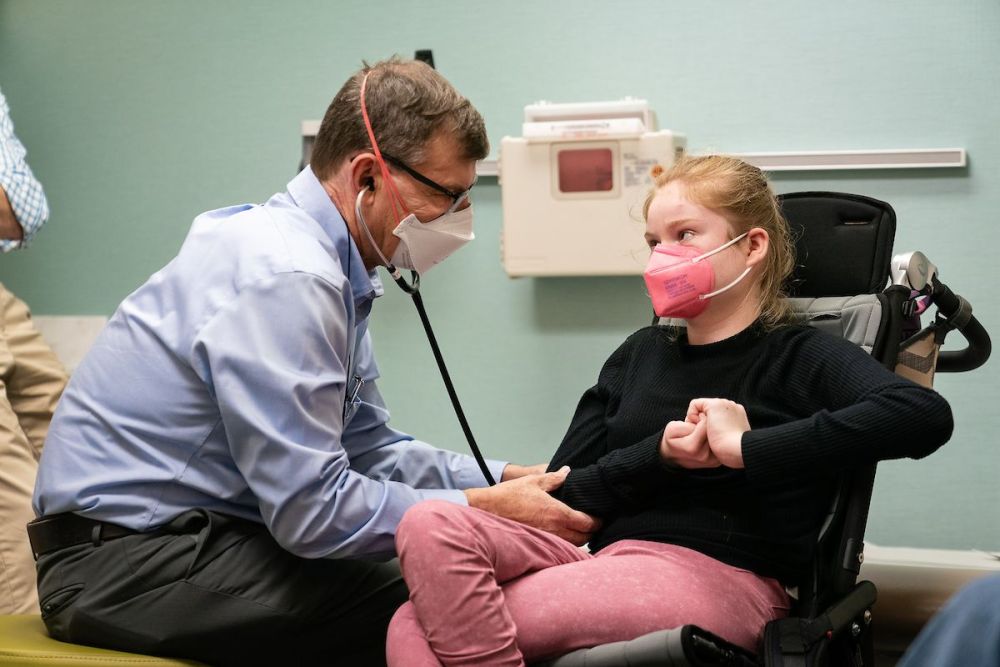
Dr. Beisang is pleased Daybue (trofinetide) is helping patients who have Rett syndrome.
Gillette is instrumental in the trials and approval of Daybue (trofinetide)
Gillette Children’s worked with the drug manufacturers, Acadian Pharmaceuticals and Neuren Pharmaceuticals, for the past 11 years and had several patients enrolled in the clinical trial of Daybue (trofinetide). Dr. Beisang and Dr. Feyma were investigators in the clinical trials of the drug which helps increase the connections between neurons and makes nerves more robust.
“The trials for Daybue started back in 2013,” Dr. Feyma recalls. “After a mountain of paperwork and hard work from our providers, research coordinators, pharmacists, prior authorization team, and others we have progress and can offer Daybue as an option.”
Dr. Beisang and Dr. Feyma are pleased there is now hope and treatment options for Rett syndrome patients, but both say this progress is the result of many years of hard work and the dedication of parents. They also stress Daybue is a treatment for Rett and not a cure.
“We consider Daybue to be the first therapy success for Rett, but it won’t be the last,” Dr. Beisang says.
“We’re inspired by our patients and families,” Dr. Feyma adds. “They are the unsung heroes. It has truly been an honor for us to work with these families and to help each child reach their potential.”
 Home Page
Home Page