
Xavier and Rose Menke are part of a new generation of children diagnosed with spinal muscular atrophy (SMA) who have a brighter future thanks to new treatment breakthroughs. In the past, children diagnosed with SMA faced a bleak future.
Research, in part done at Gillette Children’s, is now providing more positive outcomes for children diagnosed with this rare, hereditary, and often-fatal disease affecting muscle strength and movement. In the past, children diagnosed with the most common type of SMA (type 1) had a lifespan of less than 2 years without treatment.
Gillette pediatric neurologist and neuromuscular neurologist, Randal Richardson, MD, MMS, is a leading SMA researcher and has been on the forefront of developing these new treatments.

Rose, Xavier and Carrie Menke meet with Gillette neurologist, Randal Richardson, MD.
Parents appreciate the Gillette SMA care team
For the past six years, Carrie and Tony Menke have been making the four-hour drive from Sioux Falls, South Dakota to see Dr. Richardson and his neurology team.
“We just love Dr. Richardson,” Carrie exclaims. “He’s been wonderful for Xavier, who is 10, and he’s cared for our now 3-year-old daughter, Rose, even before she was born. We totally feel it’s worth the extra effort to come to Gillette in St. Paul for their treatment.”
Hope for a new generation of SMA patients
The neurology team at Gillette continues to be one of the leading pediatric facilities involved in SMA drug trials and neuromuscular disease research.
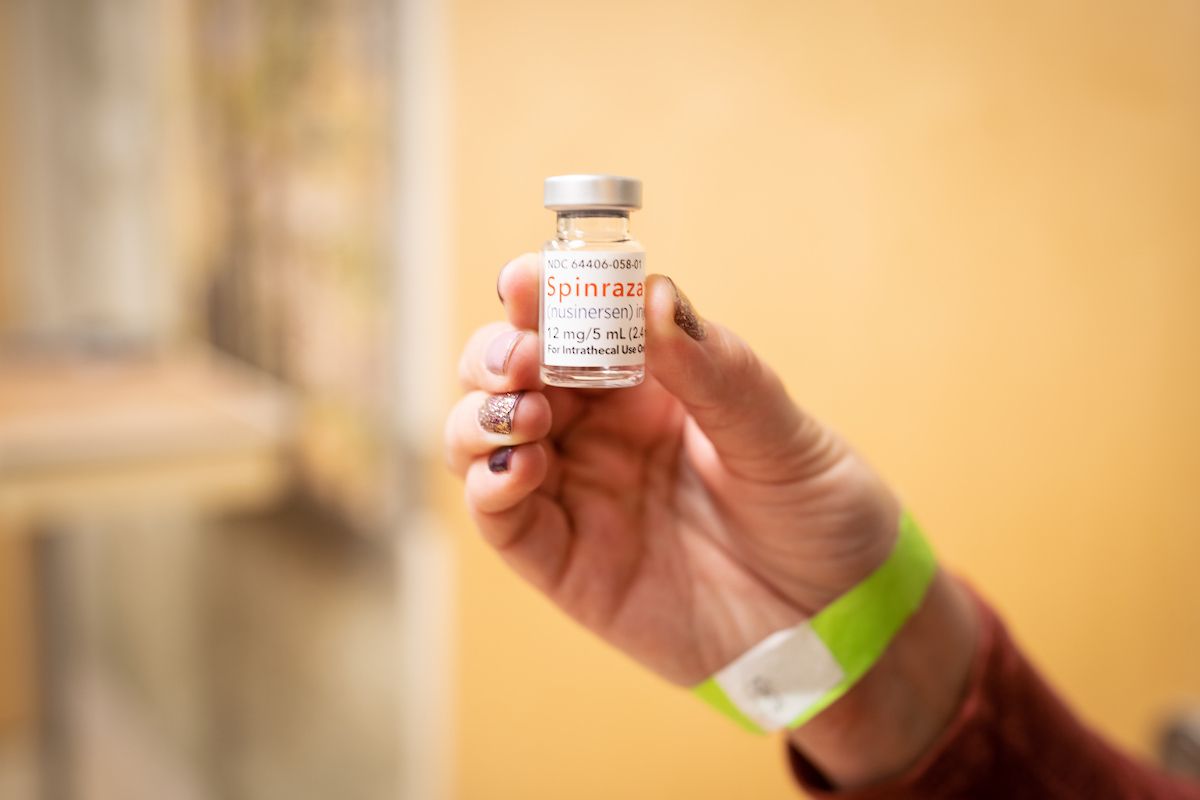
Dr. Richardson and his team were key contributors in the 2016 EMBRACE clinical trial that led to the Food and Drug Administration (FDA) to approve Spinraza ®(nusinersen) for SMA treatment.
Spinraza is hailed as the first real treatment for SMA. It works by targeting the SMN2 gene, causing it to make a more complete protein. According to Cure SMA, once a patient receives four loading doses within the first two months of treatment, they receive a maintenance dose every four months for life.
The approval of Spinraza came at just the right time for Xavier Menke who was born in November 2013.
“Xavier was officially diagnosed with SMA in 2017,” his mother, Carrie, recalls. “It was just a few months after the first SMA treatment became available.”
His parents report Xavier was born healthy, but they started noticing symptoms when he was around 2 years old.
“Something was off,” Carrie explains. “He had been toddling around and talking from an early age. He walked at 1 and was right on track. Then when he was about 2, he would often fall and using the stairs became a challenge. He started to decline rapidly.”
As Xavier’s symptoms became more severe, the Menke’s sought advice from their family pediatrician who recommended Xavier be evaluated at Gillette.
“Once we connected with Dr. Richardson, Dr. Eskuri, and the neurology team at Gillette we knew we were in good hands,” Carrie says.
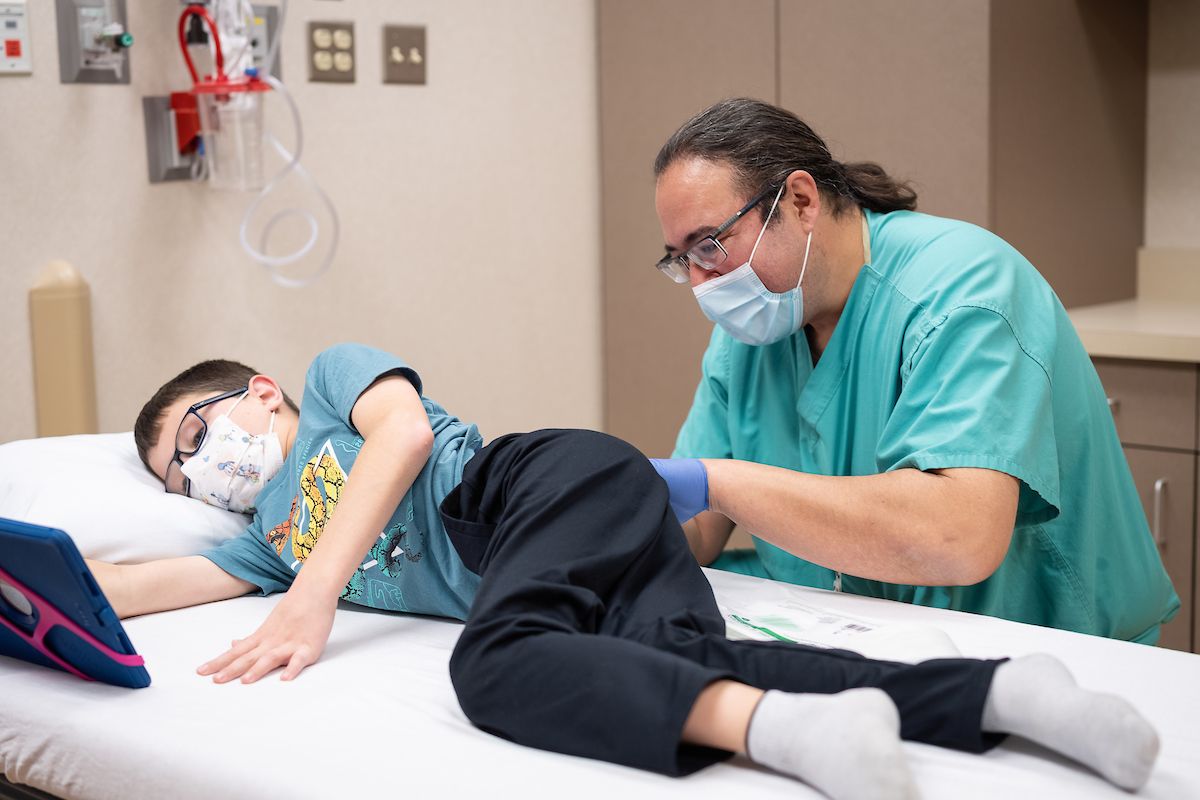
Dr. Richardson gives Xavier a dose of Spinraza during a visit to the Gillette Children's clinic.
Gillette experts make a difference in treatments
Xavier initially received the four loading doses of Spinraza and he comes to Gillette several times a year to be monitored and given lumber punctures of the medication by Dr. Richardson, who is pleased he is now able to provide additional care for SMA patients.
“Nusinersen (Spinraza) represents the first real opportunity to shift the focus in neuromuscular care from diagnostics and symptom-focused treatments to true medical treatment for a previously untreatable genetic disease,” Dr. Richardson says.
“Embracing this early and aggressively meant that I peddled a bit in hope, a word we should use more commonly in medicine,” Richardson adds. “Once the new natural history of nusinersen-treated SMA became established, well...quite pleasantly, it turned out that our hopes were indeed congruent with reality. Xavier proved to me that it is OK to hope and strive for a better tomorrow.”
The price of treatment and the promise of hope
Xavier remains remarkably calm and composed as he receives his 20th lumbar puncture of Spinraza from Dr. Richardson.
An experienced team that includes specially trained neurological nurses and a respiratory therapist assists Dr. Richardson during the procedure.
Many of these Gillette neurology team members have been working with SMA patients for years and are pioneers in how new treatments are developed and given.
“This was not easy ANY step of the way,” Dr. Richardson reflects. “Expensive medications require extensive documentation to obtain insurance approval. The actual process of treating patients with frequent lumbar punctures within an exacting treatment schedule very likely burnt out every person who had the audacity to make hope a reality.”

Gillette Children's physical and occupational therapists are part of the team monitoring and caring for Xavier Menke.
It takes a village
Dr. Richardson adds the focus and dedication of the Gillette team revolutionized the care for patients with SMA.
“Within three months of FDA approval, we were treating, and in a manner that was both efficient and patient-centered,” says Dr. Richardson.
“Creativity is another word not commonly discussed in medicine, but I do feel that every member of the team (nurses, nurse practitioners, prior authorization specialists, respiratory therapists, research, respiratory therapists, research coordinators, child life specialists, etc.) exercised their right to creatively maximize the care of these patients. In short, the entire village showed up and that made a difference.”
Xavier and his younger sister Rose, who is also diagnosed with SMA, also receive care from Gillette physical and occupational therapists who monitor their progress. A team of additional specialists look for any progression in their disease.

Rose Menke jumps during her appointment with a Gillette Children's physical therapist.
Gillette and Zolgensma help Rose
Rose Menke is part of a new generation of children diagnosed with SMA.
Before she was born, Rose was tested in utero to detect if she had SMA. The amniocentesis test came back positive for SMA, and a neonatologist was present at Rose’s birth.
Rose was born in December 2019.
“A week after Rose was born, we traveled to Gillette to see Dr. Richardson,” Carrie says.
When she was just a month-old Rose received the second SMA treatment approved by the FDA. Dr. Richardson and his team administered Zolgensma, which is the first gene therapy approved to treat neuromuscular disease.
The FDA approved Zolgensma in May of 2019. According to the Cure SMA website, Zolgensma is given through an intravenous (IV) infusion that takes about one hour. It is a one-time treatment to treat children with SMA who are younger than 2 years of age.
So far, Rose shows no sign of the symptoms associated with SMA. She walks, runs, jumps, and is a ball of positive energy.
Unlike her older brother, Xavier, Rose does not need continued lumbar punctures or have as many visits to Gillette. Rose is periodically monitored by Dr. Richardson and the neurology team to make sure she’s still doing well, and the treatment is working.
Dr. Richardson says Xavier and Rose are examples of how important it is for children to be diagnosed as early as possible and the Menke children highlight the progress in SMA care.
“Rose's continued lack of symptoms is most certainly due to Zolgensma," Dr. Richardson says.
Dr. Richardson and Gillette Children's continue to work to help patients navigate the insurance issues involved in getting coverage for the SMA treatments.
SMA treatments come with a price
The outlook for Xavier and Rose is excellent and the Menke family is grateful for Gillette and the remarkable progress in treatment options for SMA.
These new treatments come with a price—literally. According to the New York Times, Zolgensma’s list price in the United States in 2019 was $2.1 million. The January 2023 article states Zolgensma is approved for use in 46 countries and has been given to more than 2,500 children. Governments, insurance companies, and parents continue to work on ways to make this treatment affordable and possible for children.
Another New York Times article states the price of Spinraza is about $125,000 per dose. Patients initially need to have four loading doses and take three or four doses each year for the rest of their lives.
Dr. Richardson and Gillette team continue to work to help Gillette patients navigate the insurance issues involved in getting coverage for the SMA treatments. Gillette has no involvement in, or influence over the price drug companies set for Spinraza, Zolgensma, or any other SMA drug treatment. It’s important to note, both Zolgensma and Spinraza are treatment options for some, and not all, SMA patients.

Children like Xavier and Rose Menke are examples of how Gillette’s focus on research and its unwavering commitment to cutting edge care can have an impact on a child’s life.
Generations changing hands
Dr. Richardson has called the breakthroughs in SMA treatments one of the most important developments in his professional life. He has been a leader in this care revolution and, in less than 10 years, has witnessed major changes in SMA prognosis and treatment.
“There is a role for all of these medications (and more) for different patient scenarios. If treatment options exist, then most families will choose Zolgensma because of the likelihood of a one-time treatment that does not tie a family to a SMA Care Center,” Dr. Richardson says.
He adds that he thoroughly educates families about the choices they have but ultimately encourages each family to make their own treatment decisions.
“John Mellencamp once said ‘there is nothing more sad or glorious than generations changing hands.’ I did not expect alternatives to Spinraza to arise so promptly, but they did, and I did equally embrace them,” Dr. Richardson says.
The entire team of specialists at Gillette Children’s is poised for the next developments in SMA treatment and rare disease care. Children like Xavier and Rose Menke are examples of how Gillette’s focus on research and its unwavering commitment to cutting edge care can have an impact on a child’s life.
Do these symptoms sound familiar? Our 30-minute consult appointment could help get answers.
Request an appointment to connect with Gillette providers.
Meet a care team provider, find a location, learn how to get a second opinion, and more.
Gillette kids fuel our mission. You provide the spark. Donate today.



